Fundamentals involves utilizing highly technological methods such as electrochemical galvanic and electrostatic effects. These methods are employed to generate zinc oxide (Zn2+), which serves as a corrosion inhibitor. Zinc oxide can form a protective layer on the surface of metal pipes, effectively preventing oxidation, scale buildup, and water leakage.
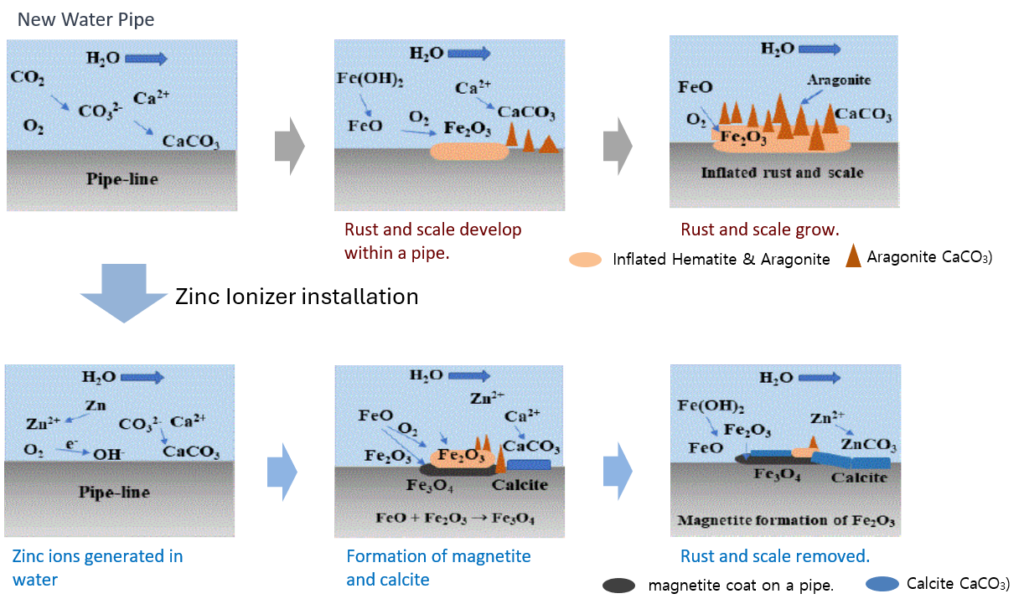
The process by which red rust, Fe2O3 (hematite), in water pipes transforms into black, dense magnetite, or Fe3O4, can be explained in several steps.
When zinc is placed in water, it releases Zn2+, which consumes dissolved oxygen (O2) in the water to produce hydrogen peroxide (H2O2). Furthermore, H2O2 is oxidized to OH–, ultimately reducing the concentration of dissolved oxygen in the water, resulting in a lowering effect.
Consequently, iron, as a piping material, is prevented from turning into FeO due to the reduced oxygen concentration in the water. Additionally, FeO already formed on the iron surface is prevented from oxidizing to Fe2O3 by O2.
FeO, which has already formed on the iron surface, reacts with red rust, Fe2O3, on the inner surface of the pipe instead of O2 in the water, transforming into Fe3O4.
Black molten Fe3O4 possesses a highly dense crystal structure and adheres to the iron metal surface as a thin film. Thus, in this sequence of changes, the release of Zn2+ by the water lowers the dissolved oxygen, gradually converting the swollen red rust, Fe2O3, into a film of Fe3O4. The Fe3O4 gradually adheres to the iron metal surface like a film, resulting in the transformation into black rust. As the process continues, rust formation ceases, and the space inside the pipe expands.
In addition to rust caused by iron in water supply pipes, other by-products besides rust are formed within the pipes due to scale, such as calcium carbonate. Research in Europe, which demonstrates relatively high hardness, mentions that zinc ions gradually cause CaCO3 crystals to disappear.
Calcium carbonate, the primary component of scale in water, primarily forms sharp aragonite crystals in water supply pipes, and these crystals coexist with Fe2O3.
Zn2+ ions inhibit the formation of CaCO3 crystals by combining with Ca2+ ions and CO3-2 ions in water, allowing Ca2+ to flow with water, thereby suppressing scale formation.
Aragonite crystals generated in the pipe are converted into orthorhombic calcite with good crystallinity by metal ions, reducing the volume of the by-product, and thereby expanding the space inside the pipe.
As Ca2+ in calcite, which has good crystallinity, is replaced by Zn2+, the calcite crystals disintegrate and break into pieces, flowing through the pipe with water currents along with silicate (SiO2) ions and slowly being discharged.