The rust suppression effect in water pipes by Zinc Ionizer
Cross-section pictures of old tap water pipe-line before and after installation of zinc ionization device and SEM images on the rust surfaces.
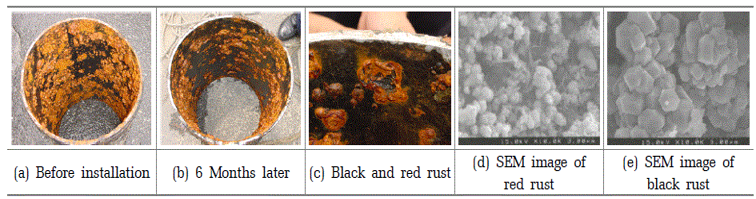
Following the installation of the zinc ionization device, the cross-section images of the tap water pipeline showed a reduction in the buildup of rust and scale deposits. The inner surface of the pipe appeared cleaner and smoother compared to before.
SEM images of the rust surfaces post-installation might reveal a change in the composition and texture. We observed a decrease in the thickness of rust layers and a transition from red/brownish colors to darker shades, indicating the formation of magnetite (Fe3O4) as a result of the zinc ionization process. The surfaces may also appear more uniform and less corroded.
The efficiency of the Zinc Ionizer
the reactions with dil. HCl solution and change of water tank in the toilet.
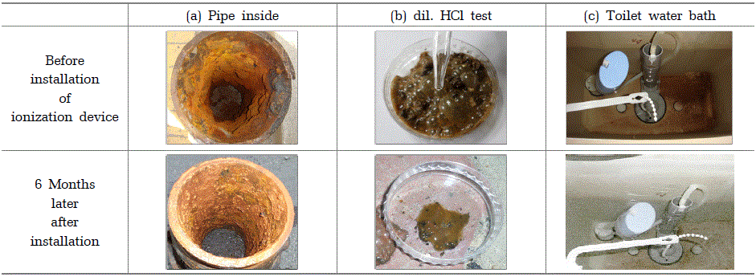
For verification purposes, a section of the pipe at the back of the ionizer was cut to reveal the interior (above) and the interior of the pipe six months later (below (a)). The interior of the pipe had corroded to a degree similar to that at the time of installation. Additionally, (b) indicates that carbon dioxide gas is produced when the ion cremator is installed and after 6 months, the corrosion products inside the pipe are removed and placed on a Petri dish, and 10% hydrochloric acid is added.
This indicates that at the time the ionizer was installed, the rust contained a significant amount of scale such as calcium carbonate, and the amount of scale decreased six months after installation. Generally, the quantity of corrosion products in the pipe varies depending on the pipe, but scale deposits such as red-rusted Fe2O3 and CaCO3 can be considered by-products in the pipe. The reduction in corrosion products after installing the ionizer could be viewed as a change brought about by the continuous flow of zinc ions, albeit at a very low concentration.
Moreover comparing the interior of the toilet water tank of the water pipe at the time of ionizer installation ((c) above) with the interior of the same water tank six months after ionizer installation ((c) below), the bottom of the water tank clearly shows, with the naked eye, a significant decrease in brown precipitate after 6 months, indicating that the red Fe2O3 precipitate, which causes rust in the pipe, has been reduced.
The efficiency of the Zinc Ionizer by examining its impact on the water flow rate
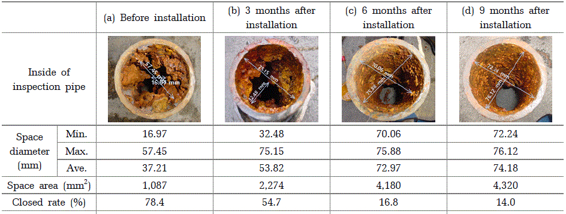
When corrosive substances such as Fe2O3 accumulate in the water supply pipe, they can cause problems with water flow. Therefore, a method of calculating the space available for water flow in the water supply pipe, known as the water flow area, was utilized. With the diameter of the water pipe being 80 ㎜, the space area within the pipe, if no corrosion has occurred, is 5,024 ㎜2.
Upon measuring the length of space before installing the zinc ionizer, the minimum was found to be 16.97 ㎜ and the maximum was 57.45 ㎜. Consequently, the area of the space within the pipe was calculated to be 1,086.9 ㎜2, resulting in a closed rate of 78.4%.
After 3 months, 6 months, and 9 months following the installation of the zinc ionizer, the length of space within the pipe increased. Consequently, the space area increased, and the closed rate decreased to 54.7%, 16.8%, and 14.0%, respectively. When viewed in terms of water flow area, compared to the 21.6% water flow rate before installing the device, it can be observed that the water flow in the pipe improved by 45.3%, 83.2%, and 86.0%.